Eczema encompasses a group of conditions that causes the skin to become itchy, rash-like, and inflammatory. Atopic dermatitis (AD), the most common type of eczema, is a chronic disease that affects about 20% of children and 10% of adults worldwide. AD occurs when the immune system becomes overactive and triggers inflammation that damages the skin. Studies have not yet shown exactly why AD develops, but those who have allergic conditions such as asthma and hay fever (or who have relatives who do) are more prone to having AD.
AD is characterized by symptoms such as erythema, where the skin turns red or purple due to inflammation, weakening of the outer part of the skin, and thickening of the skin from repeated scratching. Unfortunately, there are no cures available for any type of eczema, but there are treatments such as over-the-counter remedies, prescription topical medications, phototherapy, immunosuppressants, and biologic drugs that may reduce the severity of the disease. Most recently, Pfizer Inc.’s drug called CIBINQO has been granted marketing authorization in Great Britain as well as Japan.
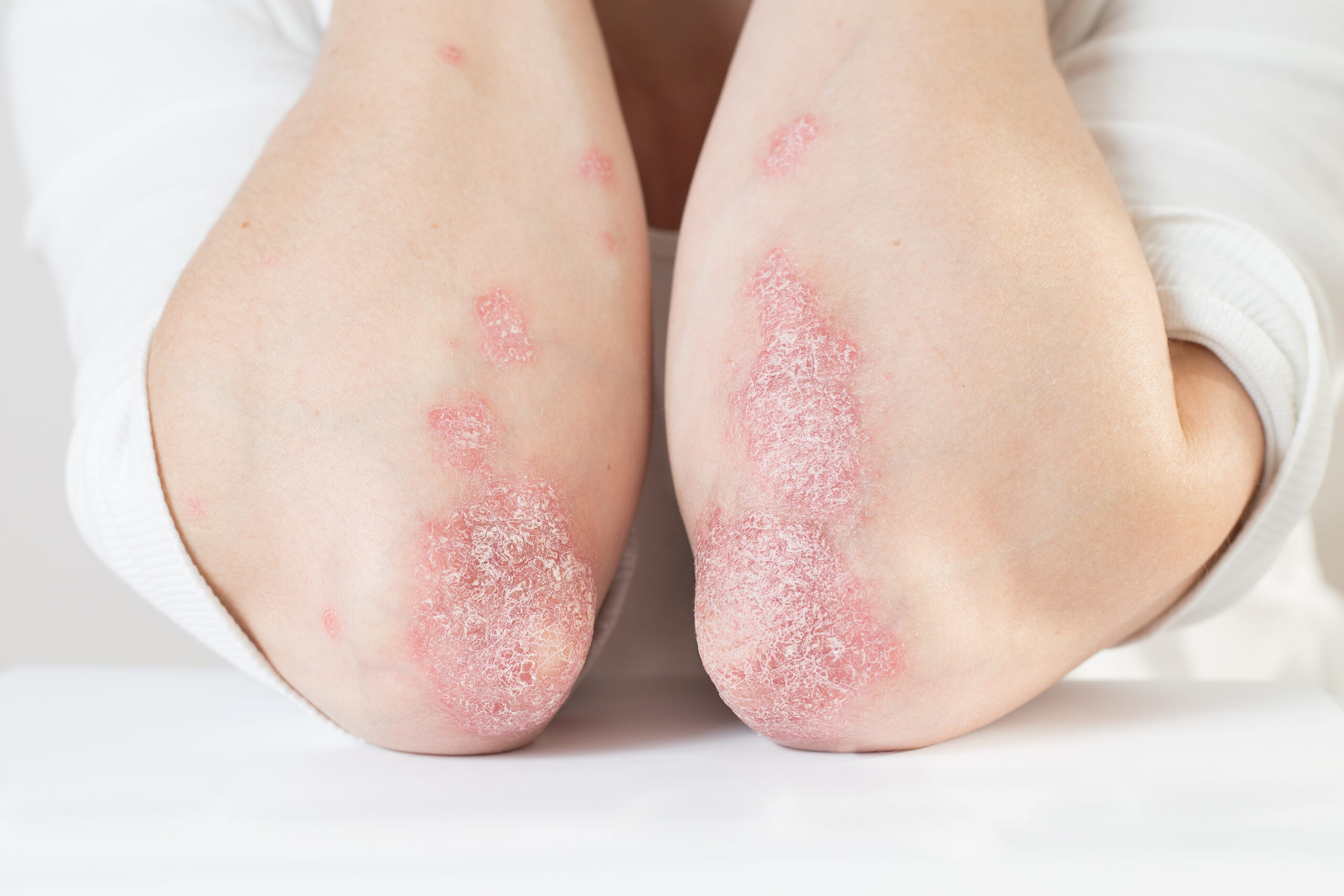
Symptoms of eczema commonly appear on the arms. Using hand creams, ointments, or prescribed topical medications are ways people can reduce the itching and rashes.
Image Source: Ulrich Zillmann
CIBINQO, also known as abrocitinib, is an oral medication taken once daily to treat moderate to severe forms of AD in both adults and adolescents 12 years and over. CIBINQO works by introducing a small molecule that inhibits Janus kinase (JAK1). JAK1 is an important protein that signals for the activation of other molecules involved in the development of AD, thus causing an inflammatory immune response. Through preventing JAK1’s normal activity, other signaling molecules that lead to AD are also inhibited, preventing the start of an eczema flare-up.
The clinical trials carried out by Pfizer Inc. showed that patients with moderate to severe AD who were treated with abrocitinib had improvements in skin clearance, disease severity, and itch. Meanwhile, those who took a placebo did not experience symptom relief, which suggested that abrocitinib had a role in the improvements. Over a shorter trial of 12-16 weeks, as well as a long-term extension study of 48 weeks, patients who received abrocitinib showed a significant reduction in AD symptoms.
Overall, such positive results led to the approval of CIBINQO in being authorized for treatment. People who are affected by severe AD typically have to undergo “systemic therapy”, which uses drugs that travel throughout the bloodstream to reach cells all over the body, similar to chemotherapy for cancer. With the development of CIBINQO, however, those with severe AD will soon have an alternate, less invasive method for treating their condition. Hopefully, the days of uncontrollable rashes from AD will be long gone!
Featured Image Source: SNAB