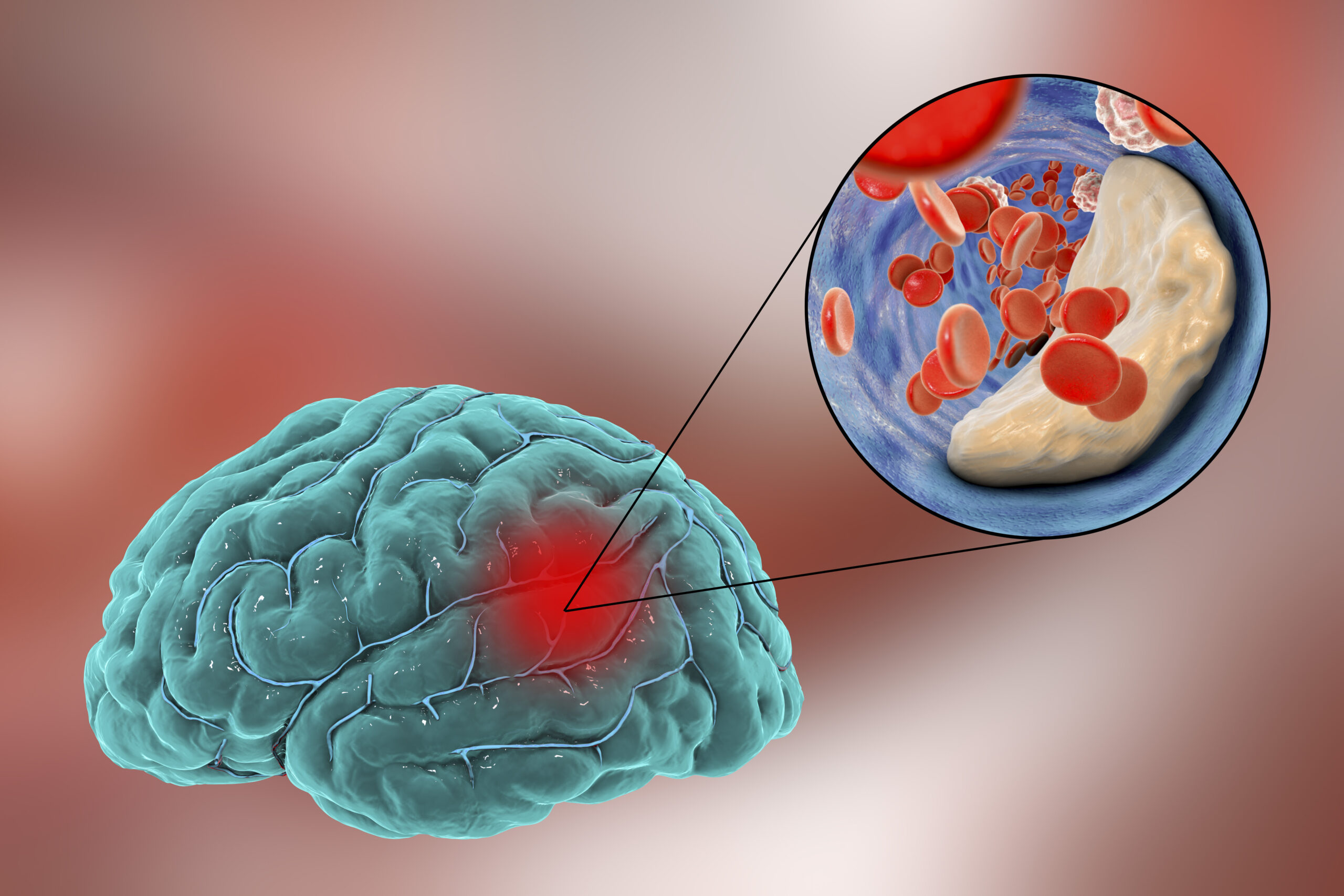
Every 40 seconds, someone in the United States has a stroke; every 4 minutes, someone dies of a stroke. Unfortunately, once you have had a stroke, you are at higher risk for another stroke. Stroke is a leading cause of death worldwide, and acute ischemic strokes (AIS) make up about 90% of all strokes. AIS occurs when a thick mass of blood–otherwise known as a clot–blocks the blood flow through an artery in the brain. This can result in partial paralysis, vision/speech problems, memory loss, emotional swings, or even personality changes.
Stroke treatment typically happens at the hospital, where a physician may prescribe a drug that breaks up the blood clots. In more critical situations where there is heavy bleeding in the brain, a neurosurgeon may perform surgical treatment. The effects of a stroke can last more than 24 hours, so patients may need rehabilitation in the form of speech or physical therapy. While some patients experience a full recovery, others may experience long-term effects or permanent loss of function depending on which part of the brain is damaged.
After a stroke occurs, patients may need to enroll in physical therapy to regain control of their limbs.
Image source: SDI Productions.
Because of the frequency and severity of strokes in the population, measures have been taken to develop new treatments for the condition. Around mid-2021, the FDA approved its first nerve stimulation device to help those suffering from the lasting effects of stroke to regain control of their limbs. The medical device company MicroTransponder developed the Vivistim system that includes an implantable pulse generator placed under the skin with electrodes connected to the vagus nerve, which connects the brain to the heart and lungs. During rehabilitation, healthcare providers or patients at home can stimulate the device by holding a magnet over it. The device then sends electrical pulses to the nerve to help retrain the brain in responding to motor signals to help move muscles. In clinical trials, treated patients saw significant improvement in motor function compared to those without the nerve stimulation device.
More recently, the medical device company BrainsGate developed the Ischemic Stroke System. With this device, electrodes are implanted in the mouth to deliver electrical stimulation to the sphenopalatine ganglion (SPG), a group of nerve cells linked to sensation and pain. Pre-clinical studies had shown that SPG stimulation led to increased cerebral blood flow and reduced damage to the brain after stroke. During clinical trials, however, potential negative side effects included airway endangerment, chronic pain from the implantation site, risk of brain swelling, and ultimately, uncertainty of the study’s results. BrainsGate’s device was rejected from FDA approval, but the usage of implantation devices continues to be investigated for AIS treatment. Expanding the purpose of implant technology thus opens the path to more treatment options and non-drug interventions for patients suffering from the most common strokes.
Featured Image Source: Kateryna_Kon